
PRODUCT DEVELOPMENT ENGINEERING
Transforming new technologies into innovative products for 20 years.
Complete product development engineering services from concept to launch.
A world-class engineering team available on-demand.
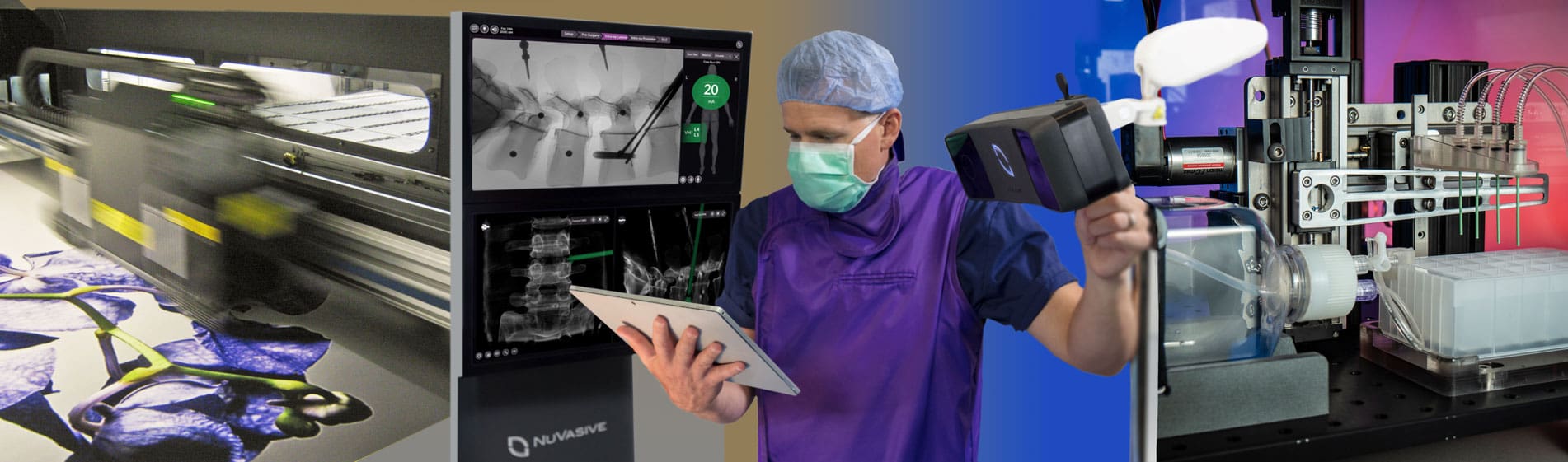
EXPERIENCE
HUNDREDS OF PRODUCTS, MULTIPLE INDUSTRIES
A seasoned team of developers for complex systems, low to high volume products, a broad range of technologies, devices, instruments, and consumables.

INNOVATION
INNOVATORS, CREATORS, PROBLEM SOLVERS
Experienced enough to know if there is an existing solution, and creative enough to develop novel solutions when there isn’t. NOVO means innovation.

PERFORMANCE
CONSISTENTLY EXCEEDING EXPECTATIONS
Teamwork perfected by hundreds of projects, extensive prototyping and testing facilities, and a product development process that drives convergence produces results you can rely on.
EXPERIENCE
HUNDREDS OF PRODUCTS, MULTIPLE INDUSTRIES
A seasoned team of developers for complex systems, low to high volume products, a broad range of technologies, devices, instruments, and consumables.

INNOVATION
INNOVATORS, CREATORS, PROBLEM SOLVERS
Experienced enough to know if there is an existing solution, and creative enough to develop novel solutions when there isn’t. NOVO means innovation.

PERFORMANCE
CONSISTENTLY EXCEEDING EXPECTATIONS
Teamwork perfected by hundreds of projects, extensive prototyping and testing facilities, and a product development process that drives convergence produces results you can rely on.























Services
NOVO is an ISO 13485 medical device developer with experience in a wide range of device categories, including on-body injectors, catheters, insulin pumps, CGM systems, medical device software, and more.
MEDICAL DEVICE DEVELOPMENT
SEE WHAT'S POSSIBLEOur hardware and software developers have extensive industry credentials with major biotech product companies. We are conversant with the development of instrument-and-consumables products as well as lab automation applications. NOVO combines biotech industry expertise with high-volume, high-performance, low-cost design methodologies from other industries.
BIOTECH INSTRUMENT DEVELOPMENT
SEE WHAT'S POSSIBLE3D
AND INKJET PRINTER DEVELOPMENT
Many of our design engineering experts hail from the inkjet printer industry and have since become experts in 3D printer development. Our understanding of the physics operation and systems engineering challenges in these complex, high-performance products is extensive, and we have yet to encounter a printer development problem we were unable to solve.
SEE WHAT'S POSSIBLEMany of our design engineering experts hail from the inkjet printer industry and have since become experts in 3D printer development. Our understanding of the physics operation and systems engineering challenges in these complex, high-performance products is extensive, and we have yet to encounter a printer development problem we were unable to solve.
AND INKJET PRINTER DEVELOPMENT
SEE WHAT'S POSSIBLE3D
3D
AND INKJET PRINTER DEVELOPMENT
COMMERCIAL PRODUCT DEVELOPMENT
The product development skills we have acquired on medical, biotech, and printer projects make us well-suited to more technical commercial product development efforts. For a commercial product development effort involving new technologies or manufacturing processes, a high level of complexity, or sensitive intellectual property, NOVO is an excellent partner.
SEE WHAT'S POSSIBLECUSTOM AUTOMATION
Our engineers have created custom automated test equipment for medical devices, stand-alone lab automation solutions, complete workcells, and process automation for both R&D and manufacturing environments. NOVO’s hardware and software expertise and our culture of innovation make us the natural choice for tough automation problems.
SEE WHAT'S POSSIBLEPRE-PRODUCTION MANUFACTURING
NOVO maintains a dedicated assembly area for controlled manufacturing builds of products under development. These builds are used for verification testing, clinical trials, and product validation activities. Performing initial pilot manufacturing in the same location as product development provides a number of key benefits to our clients.
SEE WHAT'S POSSIBLEFeatured Case Studies
Tethered Sensor Platform
Tethered Sensor Platform System Description Planck Aerosystems has developed a platform for deploying sensor systems mounted to autonomous aircraft operating from a moving vehicle. They refer to it as the AVEM™ tethered sensor platform. Its purpose is to free the operator from the need to pilot the aircraft so the operator can maintain focus on other mission critical activities. The use of a tether to pass both power and data eliminates RF communications and permits nearly unlimited deployment duration. The system is designed for autonomous operation using Planck's Autonomous Control Engine (ACE™). This system controls all in-flight operations including vehicle tracking, altitude
READ MORE SEE PORTFOLIOOral Biopharmaceutical Delivery
A Capsule-based, Oral Drug Delivery Platform NOVO is pleased to be an engineering design and development partner to Progenity for the development of their oral biopharmaceutical delivery system (OBDS). The OBDS is a drug delivery platform, configured as a capsule, for the delivery of large-molecule therapies. The capsule delivers a liquid formulation of the drug directly to the small intestine for optimal uptake. The delivery system is needle-free. The OBDS platform is suitable for the delivery of a range of molecules including monoclonal antibodies, peptides, and nucleic acids. Miniaturization of the electromechanical systems, and the means to localize to the desired location
READ MORE SEE PORTFOLIOSmart Pen Cap for Insulin Management
Engineering a Seamless Diabetes Experience Bigfoot Unity diabetes management system Client Need & Project Overview Today, connected medical devices are expected to simplify treatment decisions and support real-time data sharing. When Bigfoot Biomedical began developing the Unity™ Diabetes Management System in 2021, those expectations were just beginning to take shape. Unity was built around familiar tools such as disposable insulin pens, continuous glucose monitors, and smartphones to provide an intuitive, supportive insulin dosing experience. As Bigfoot puts it, the system helps answer a critical daily question: “How much insulin would my doctor recommend I take right now?” To bring this vision
READ MORE SEE PORTFOLIOSTRATASYS J55 3D PRINTER DEVELOPMENT
NOVO is proud to have been an engineering resource for Stratasys during their groundbreaking Stratasys J55 3D printer development. We are now a beta tester for the J55 and we're using it on a daily basis to create prototypes for our clients. We take our hats off to Stratasys for introducing another groundbreaking 3D printer product that advances the state-of-the-art for practical in-house prototyping. J55 3D Printer Development Background Stratasys is one of the pioneers of the 3D printing industry. Their first 3D printer based on fused deposition modeling (FDM) technology was launched in 1992. Stratasys has steadily refined and expanded their product
READ MORE SEE PORTFOLIOSingle-Use CGM Auto-Applicator Development
Development of the G6 Applicator Project Scope DexCom G6 Applicator NOVO performed initial concept development, feasibility prototypes, and early-stage development for DexCom’s auto-applicator. The auto-applicator is the sensor delivery device for their G6 continuous glucose monitoring (CGM) system. When applied to the body and triggered by the user, the single-use applicator automatically inserts a sensor subcutaneously, makes the electrical connection, and adheres the transmitter base to the patient. The applicator for DexCom’s G5 and earlier CGM generations, also co-developed by NOVO, has been on the market for over a decade. Technical Challenges NOVO performed system modeling to simulate the delivery cycle
READ MORE SEE PORTFOLIOBenchtop Synthetic Biology Workstation
Creating the World’s First DNA Printer Client Need & Project Overview Telesis Bio BioXP 3250 benchtop synthetic biology workstation The ability to synthesize DNA and mRNA on demand has become a cornerstone of modern synthetic biology, fueling rapid advances in vaccines, immunotherapies, and protein engineering. Long before today’s surge in automated DNA synthesis and lab-on-demand platforms, Synthetic Genomics (SGI) envisioned a benchtop synthetic biology workstation, essentially a DNA printer, that could assemble DNA fragments into longer molecules in a compact, automated format. SGI selected NOVO as a design and engineering partner for the BioXp 3200, the world’s first commercial benchtop synthetic biology workstation. The
READ MORE SEE PORTFOLIOInsulin Pump Controller Development
INSULIN PUMP CONTROLLER DEVELOPMENT FOR AN AUTOMATED INSULIN DELIVERY SYSTEM Insulin pump controller, CGM, and mobile device Bigfoot Biomedical, a high-profile startup on a mission to improve the lives of Type 1 diabetics (T1D), selected NOVO to develop the insulin pump controller for their automated insulin delivery system. After observing family members struggle to manage the disease, one of Bigfoot’s founders, Bryan Mazlish, recognized the need for a system that would reduce the cognitive burden on diabetic patients and their caregivers—an automated system to perform glucose measurement and automate the medication administration. He also recognized that the
READ MORE SEE PORTFOLIOPAGE-WIDE-ARRAY INKJET PRINTERS:
NOVO was a major development partner for the first company to commercialize a page-wide-array printer—a significant advancement in inkjet printing technology. The client company core competency was in CMOS/MEMS and they had developed an innovative low-cost thermal inkjet printhead suitable for use in multiple printer applications. Lacking expertise in printer design, testing, and commercialization, they chose NOVO as a long-term development partner and worked with NOVO for over five years. NOVO engineers worked closely with the client’s printhead team to develop printers for various market segments, including office, photo, label, and industrial printing. Page-wide-array printheads offer the major simplification of eliminating
READ MORE SEE PORTFOLIORecent Posts
-
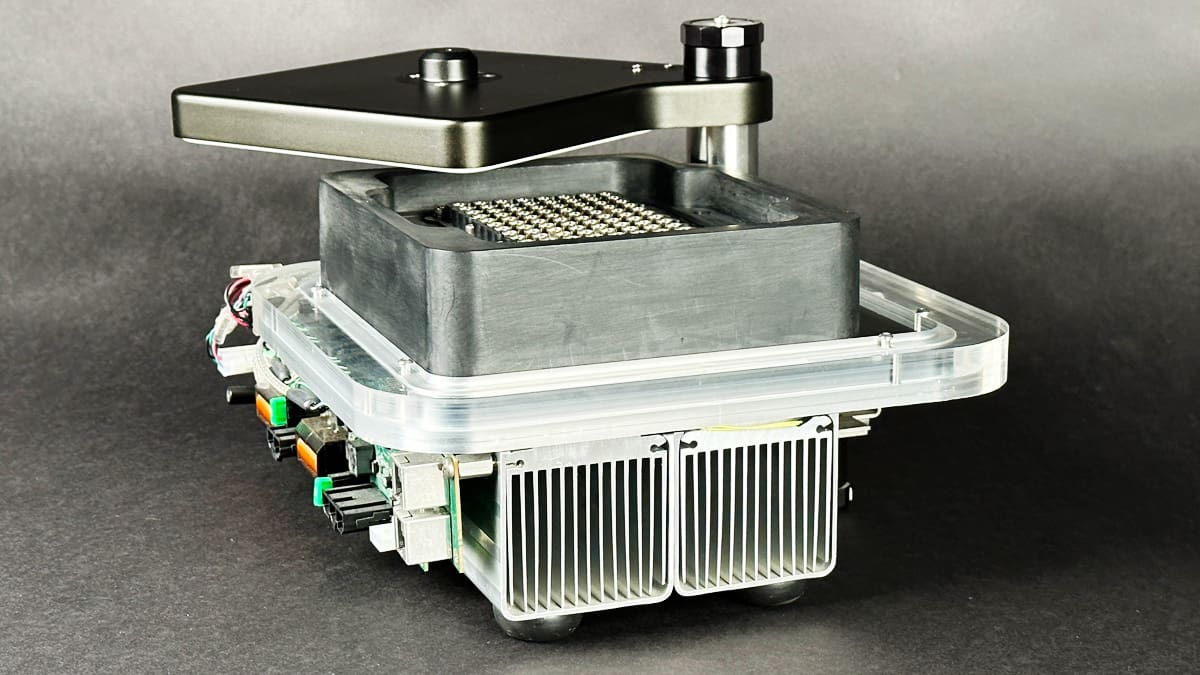
Deterministic Analysis of Thermoelectric Cooling Systems in Thermal Cycling Applications
Read MoreAbstract Thermoelectric coolers (TECs), also known as Peltier devices or thermoelectric heat pumps, offer numerous advantages, including the ability to heat and cool from a single device, bi-directional operation,…
: Deterministic Analysis of Thermoelectric Cooling Systems in Thermal Cycling Applications -
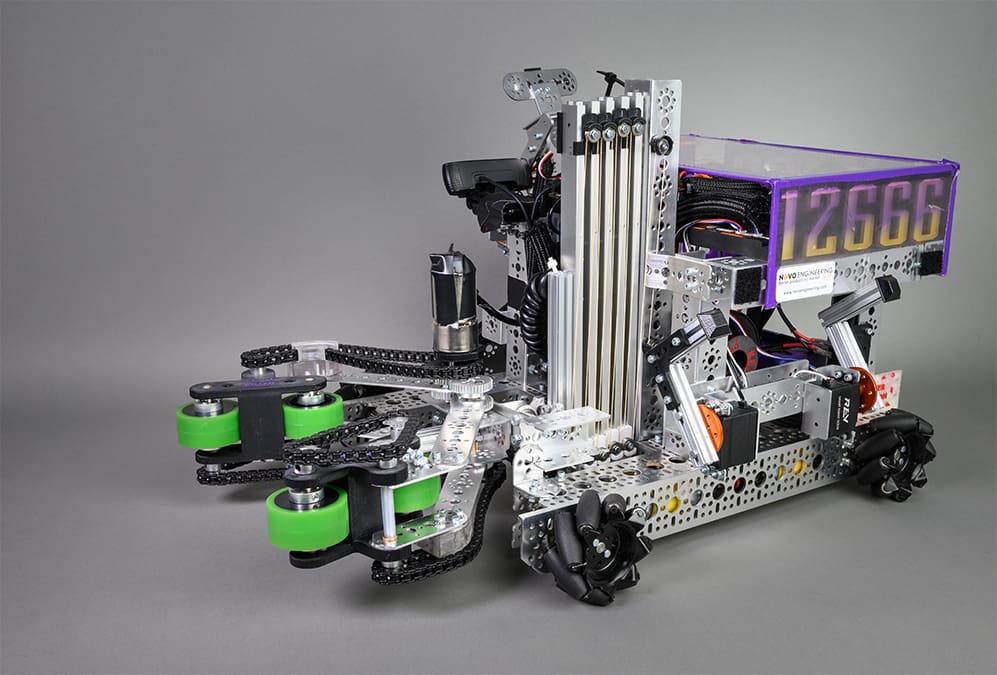
Support for Youth Robotics Competitions
Read MoreNOVO Engineers provide support for youth robotics competitions, like the First Robotics programs, as a way of sharing their engineering expertise with students and introducing them to the process…
: Support for Youth Robotics Competitions -

The Transcend Micro Travel CPAP
Read MoreObstructive sleep apnea is a common malady in the U.S. and world wide. This condition makes it difficult to get a good night’s sleep. Continuous positive airway pressure (CPAP)…
: The Transcend Micro Travel CPAP
