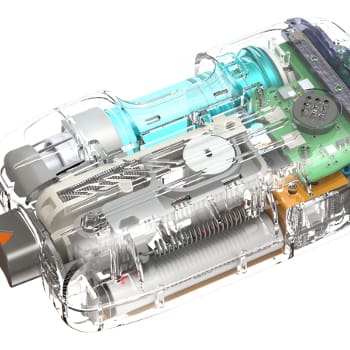
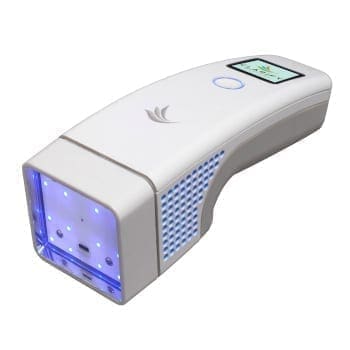
RUGGEDIZING A CLASS III MEDICAL DEVICE FOR SUBMERSION AND ESD
In addition to safety requirements related to creepage and clearance, a Class III medical device needed to pass IPX-7 rating. This device was a durable to be used by patients, and had an exposed connection. NOVO’s senior engineers designed an innovative, custom solution to meet both safety and water ingress protection requirements by utilizing technology rare for this type of device. The final product passed clinical trials and was recently introduced to the market.
Benefits to the Client:
- In-house manufacturing and assembly: the solution NOVO designed allowed the device to be manufactured and assembled in house, which saves the client untold amounts of money in shipping over years of manufacturing. In addition, some internal components were delicate, so eliminating shipping also reduces costs indirectly by decreasing the risk of broken and damaged parts.
- Part reduction: NOVO’s design reduced the overall number of parts, again saving the client money by decreasing the opportunity for mechanical failures and the chances of parts not being available long term.