Optimizing a Phototherapy Device for Ease of Use
Ultra-violet (UV) phototherapy for skin conditions such as vitiligo, eczema, and psoriasis has traditionally been administered by a healthcare provider (HCP) in a clinical setting. NOVO was approached by Clarify Medical to provide comprehensive product development services for a phototherapy device with improved ease of use that would allow it to become the first phototherapy system to use a mobile application as the user interface. The client, a startup running on investment capital, placed a high priority on bringing a strong product to market quickly.
Phototherapy System Design Challenges
Mechanical Design
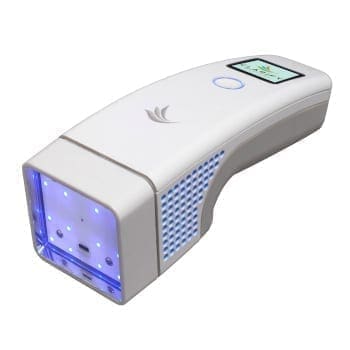
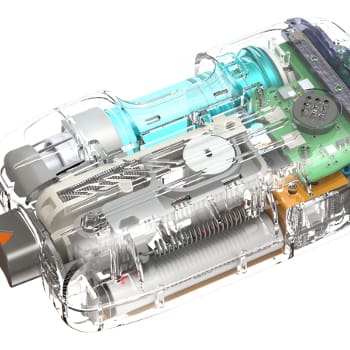
One of the most significant challenges on this project was the use of UV LEDs, a first for a device of this kind. UV LEDs have a narrow band emission spectrum, and it was necessary to find narrow-band UVB LEDs with a peak wavelength as close as possible to the optimal therapeutic wavelength to avoid erythemic response. To optimize efficiency and achieve the peak wavelength, the client contracted directly with the supplier to co-develop the LEDs.
UV LEDs are not power-efficient, and because of the large amount of heat generated by the LEDs, the ability to remove heat was the primary limitation of the system. Dissipation of heat out of the die of the LED was critical to LED performance; also, the device needed to meet temperature requirements to comply with IEC 60601-1-11 standards for home-use medical devices. NOVO used a metal-core PCB to pull the heat out of the LED as efficiently as possible and used a fan to actively pull the heat away from the metal-core PCB.
Hardware features that facilitated home use were the industrial design, small overall size and weight, and battery operation.
Electronics and Firmware
The complete system comprised a handheld device with a Bluetooth Low-Energy (BLE) connection to a smartphone application. With a smartphone as the primary user interface, this phototherapy device would be much easier to use than others already on the market. NOVO built the electronics hardware design around a Bluetooth module with power management, LED current control, and safety circuits. The handheld device also had a small organic LED (OLED) display for which NOVO implemented screen designs in the firmware to guide the user through operation of the device. NOVO built all firmware from the ground up, including specifying the application programming interface (API) to the mobile application, the Bluetooth communications, prescription definition, treatment process flow, system alerts, data logging, security policy proposal, and firmware over-air updates.
Project Delivery
NOVO delivered full product design with verification evidence and reports for release in the client’s quality management system (QMS). The client completed a 510k submission to the FDA in early 2017, and the device was released for commercial production late that year. In addition to the use of the smartphone application, other differentiators for this product were the precision and uniformity of the treatment. Ease of use was facilitated by the well-defined treatment area, consistent placement of the device relative to the treatment surface, and indicators for treatment duration.